The ETC and ATP synthesis are coupled by a proton gradient across the inner mitochondrial membrane. The transfer of electrons through the respiratory chain leads to the pumping of protons out of the matrix. Protons flow back into the matrix through ATP synthase, releasing energy that is used to make ATP.
ATP Synthase
ATP synthase is composed of two subunits, F0 and F1. The 0 subunit is embedded in the inner membrane, and 1 subunit, containing the catalytic parts, protrudes into the matrix. The 1 subunit consists of five polypeptide chains, alpha (a), beta (b), gamma (g), delta (d), and epsilon (e). The a and b chains which make up the bulk of 1 are arranged alternatively in a hexameric ring. Both a and b bind nucleotides, but only b participates in catalysis. The g subunit is a long helical coiled coil that extends into the middle of the a/b hexamer. The g subunit breaks the symmetry of the a/b hexamer, because each monomer interacts with a different face of the g subunit. The 0 subunit contains the proton channel. An "a" (distinct from a=alpha) subunit binds to 0. The 0 and 1 units are connected by the g/e stalk and by an exterior column made up of the "a" subunit, two "b" (distinct from b=beta) subunits, and the d subunit.
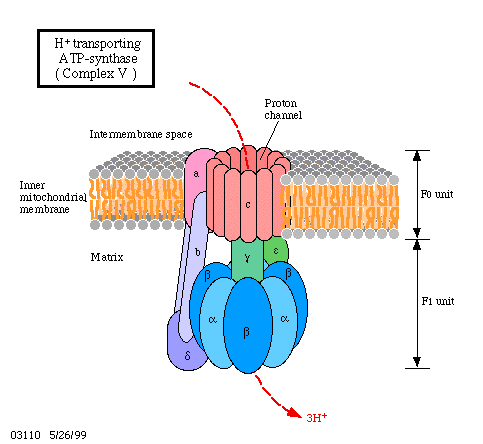
Unexpectedly, experiments have shown that enzyme bound-ATP forms in the absence of a proton-motive force. However, ATP does not leave the catalytic site unless protons flow through the enzyme. It was proposed that each b subunit can perform a different step in ATP synthesis. For example, (1) ADP and Pi binding, (2) ATP synthesis, and (3) ATP release. Rotation of the g subunit drives the interconversion of each b position. Each subunit will progress from T --> O --> L, and no two subunits will ever be in the same conformation.
Proton Flow Powering ATP Synthesis
The "a" and "c" subunits are involved in the movement of protons. The "a" subunit has two half channels (cytoplasmic and matrix). Additionally, each polypeptide chain on the "c" subunit has an aspartate residue that will bind the proton. The aspartate residue will bind a proton entering the cytoplasmic half channel of the "a" subunit and will hold on to the proton and rotate until the proton is in a proton poor environment and leaves through the matrix half channel. The movement of protons through the half channels from the high proton concentrations of the cytoplasm to the low proton concentration of the matrix powers the rotation of the "c" ring. The "c" ring is tightly linked to the g and e subunits, thus as the "c" ring turns, the g and e units turn inside the a/b hexamer. The two "b" chains and the d subunit keep the a/b hexamer from rotating. Each 360 degree rotation leads to the synthesis of 3 molecules of ATP. Thus, if there are 10 "c" subunits therefore, each ATP generated requires the transport of 10/3 = 3.33 protons.
Mitochondrial Shuttles
The respiratory chain regenerates NAD for use in glycolysis. NADH from glycolysis cannot simply cross the inner membrane, therefore, the electrons from NADH, rather than NADH itself, are carried across the membrane. There are two different types of shuttles that transfer electrons from NADH to the ETC.
The Glycerol-3-Phosphate Shuttle An electron pair from NADH is transferred to dihydroxyacetone phosphate (glycolytic intermediate) to form glycerol-3-phosphate. Glycerol-3-phosphate is reoxidized to DHAP on the outer surface of the inner mitochondrial membrane, where an electron pair is from G3P is transferred to FAD prosthetic group. The FADH2 transfers its electrons to coenzyme Q which enters the ETC at complex II. Notice that FADH2 enters the ETC, thus 1.5 rather than 2.5 ATPs are produced. The glycerol-3-phosphate shuttle is especially prominent in muscle. | Malate-Aspartate Shuttle In the heart and liver, electrons from cytoplasmic NADH are brought into mitochondria by the malate-aspartate shuttle, which uses two membrane carriers and four enzymes. |
How does ATP leave the mitochondria?
A specific transport protein, ATP-ADP Translocase, enables these molecules to transverse the inner membrane. ADP enters the mitochondria only if ATP exits. The inhibition of ATP-ADP translocase leads to inhibition of cellular respiration.
Regulation of Cellular Respiration
Total Yield of ATP per molecule of glucose
Glycolysis- 2 ATP, 2 NADH
Linking Step- 2 NADH
Krebs Cycle- 2 ATP, 6 NADH, 2 FADH2
Electron Transport Chain- 26 ATP
TOTAL ATP = 30
Note that if the malate-aspartate shuttle was used, 2 more ATP could be generated.
Electrons do not usually flow through the ETC to oxygen unless ADP is simultaneously phosphorylated. What ADP concentration rises, such as in active muscle, the rate of oxidative phosphorylation will increase to meet the needs of the muscle. The level of ADP also affects the rate of the TCA cycle. As ADP levels decrease, the TCA cycle slows because there is no NAD or FAD to feed the cycle. As ADP levels rise and the ETC speeds up, NADH and FADH2 are oxidized and the TCA cycle becomes more active.
Uncoupling of ETC
ETC can be uncoupled from ATP synthesis to generate heat. UCP-1 is an uncoupling protein that forms a pathway for the flow of protons from the cytoplasm to the matrix. Therefore, the energy of the proton gradient normally used to make ATP is shortcircuited, and heat is released as protons move through. This pathways is activated when core body temperature begins to fall. In addition, fatty acids are released in response to a temperature drop, and they activate thermogenin.
Poisons that inhibit ETC
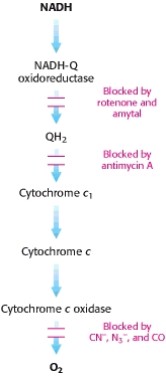
Oligomycin inhibits ATP synthase causing the electron transport chain to stop
2,4-dinitrophenol uncouples the ETC from ATP synthesis
ATP-ADP translocase is inhibited by atractyloside and bongkrekic acid causing oxidative phosphorylation to stop because no ADP is being brought into the cell.
Berg, J., Tymoczko, J., Stryer, L. Biochemistry 6E. ©2007 W.H. Freeman and Company